One of the most pressing environmental problems over the last century has been the depletion of the ozone layer. But what is the ozone layer, and why does it matter?
Ozone is a gas present naturally within Earth’s atmosphere. It is formed of three oxygen atoms (giving it the chemical formula O3). Its structure makes it unstable: it can be easily formed and broken down through interaction with other compounds.
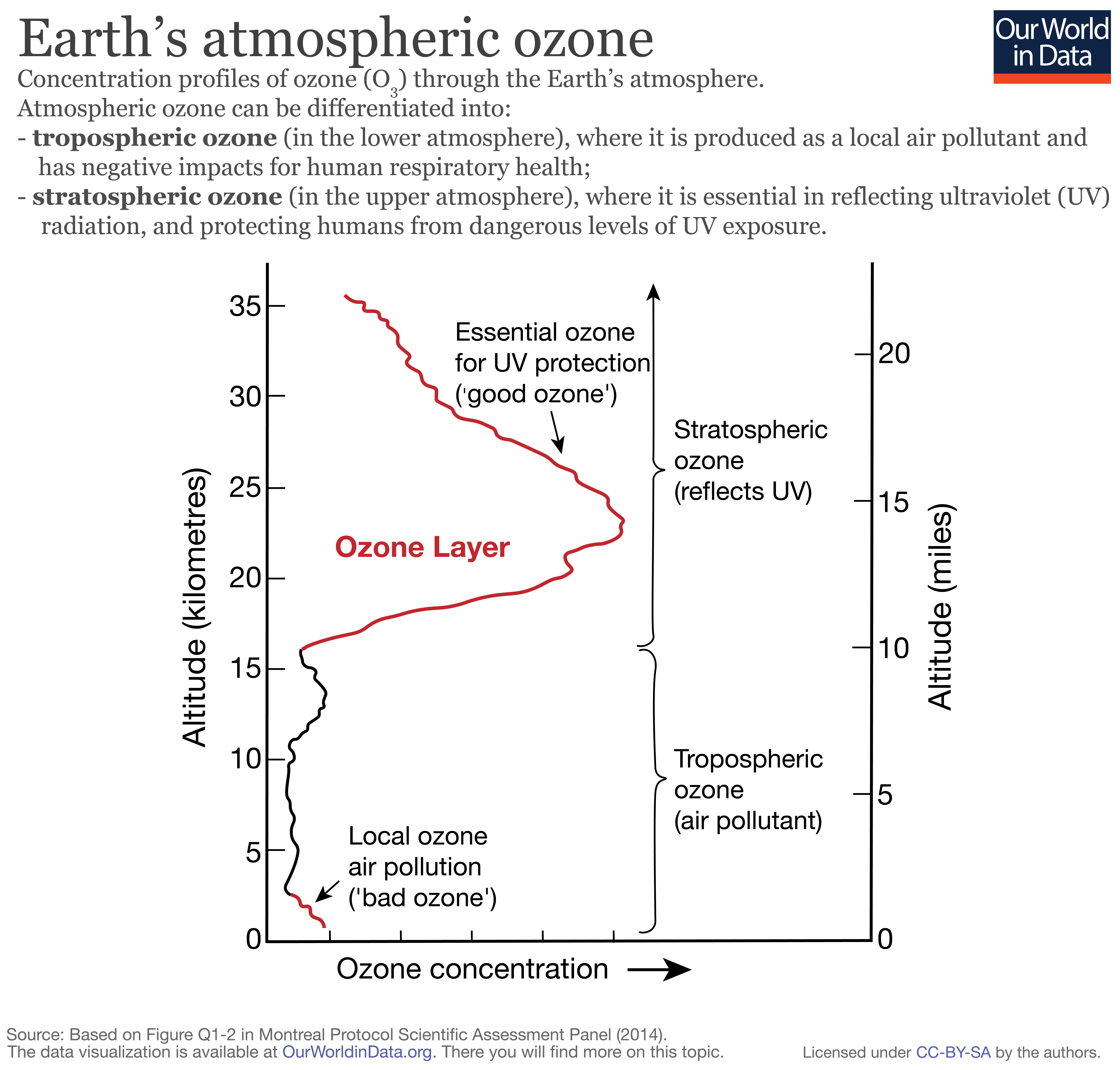
Ozone is most highly concentrated at two very different altitudes in the atmosphere: near the surface, and high in the atmosphere (in the stratosphere). Its function is very different in these two zones.
‘Good ozone’ and ‘bad ozone’
Ozone close to the surface is called tropospheric ozone, and it is often referred to as ‘bad ozone’. Ozone concentrations are lower in the troposphere than in the stratosphere.
We can see this in the diagram.
However, ozone concentrations close to the Earth’s surface can be temporarily and locally higher, because of emissions from motor vehicle exhausts, industrial processes, electric utilities, and chemical solvents. Ground-level ozone is a local air pollutant, and can negatively impact human health. Breathing ozone is particularly harmful to the young, elderly, and people with underlying respiratory problems.
This is very different from ozone high in the atmosphere: stratospheric ozone. It’s referred to as ‘good ozone’.
As shown in the diagram, ozone concentrations are higher in the stratosphere than in the troposphere.
The stratosphere includes the zone commonly called the ‘ozone layer’. It plays a crucial role in keeping the planet habitable by absorbing potentially dangerous ultraviolet (UV-B) radiation from the sun. Before its depletion, the ozone layer typically absorbed 97 to 99% of incoming UV-B radiation.
This means we need high ozone concentrations in the stratosphere to ensure that life — including human life — is not exposed to harmful concentrations of UV-B radiation.
In our work on the ozone layer, we focus on this ozone high in the atmosphere (the ‘good ozone’). The impact of ozone near the surface (‘bad ozone’) is covered in our work on air pollution.
Why is the ozone layer important?
The ozone layer absorbs 97% to 99% of the sun’s incoming ultraviolet radiation (UV-B).
This is fundamental to protecting life on Earth’s surface from exposure to harmful levels of this radiation, which can damage and disrupt DNA.
In the 1970s and ‘80s, humans emitted large amounts of gases that depleted this ozone in the upper atmosphere. As ozone concentrations in the stratosphere fell, and a hole in the ozone layer opened up, there have been measurable increases in the amount of UV-B radiation reaching the surface.
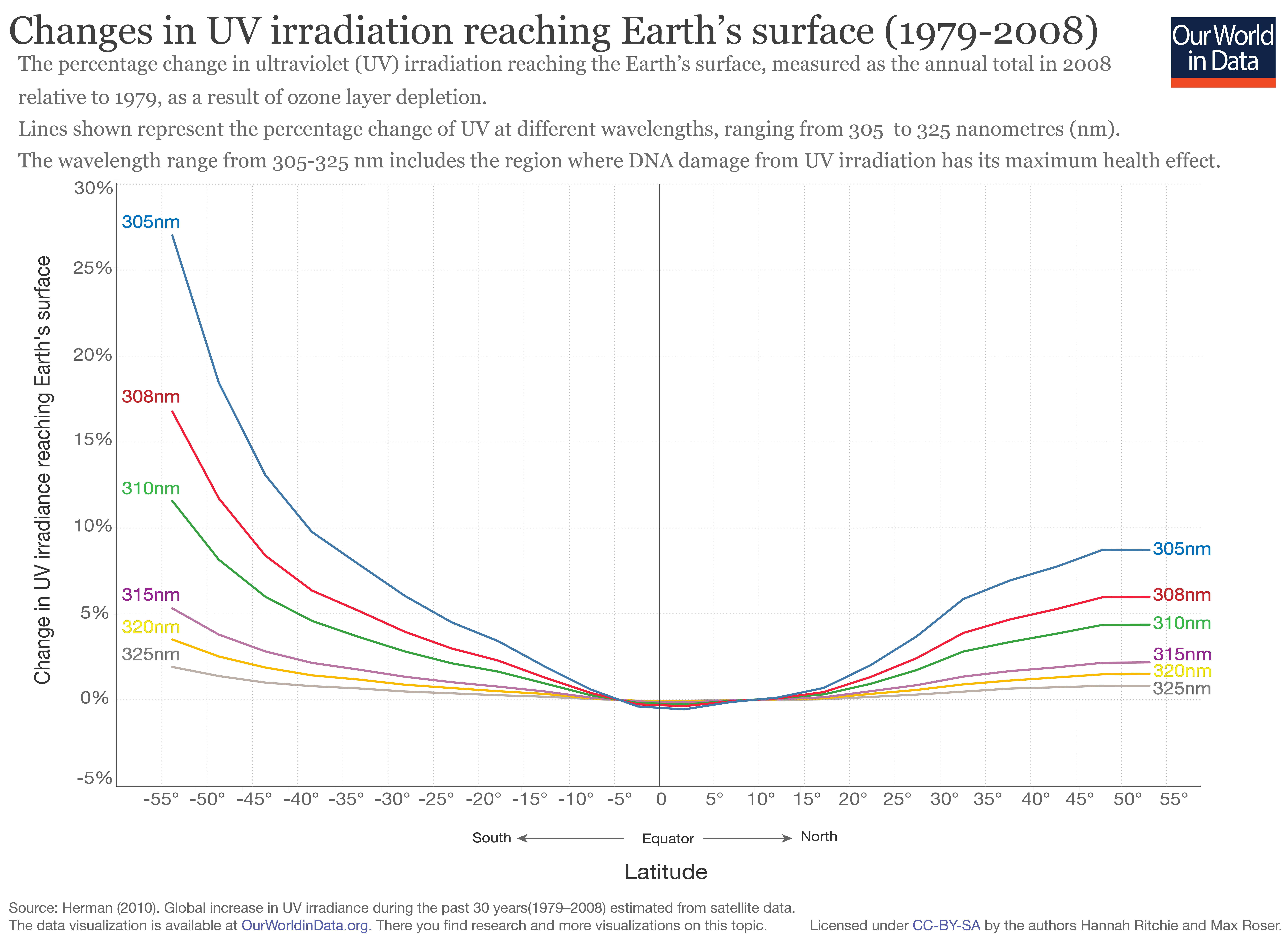
The chart shows the measured change in annual quantities of UV irradiance reaching Earth’s surface, in 2008 compared to 1979.1
What’s noticeable is that ozone depletion and UV irradiance have increased much more in the Southern Hemisphere. This is because ozone depletion is also impacted by temperature and sunlight. Temperatures are colder at high latitudes in the Southern Hemisphere, so polar stratospheric clouds can form. These clouds can accelerate the reactions that break ozone down.
You will also notice that ozone depletion is worse at higher latitudes. It’s non-existent at the equator, and rises steeply towards the poles. Again, this is influenced by temperature and sunlight. That’s why ozone holes form at the poles, rather than the equator.
This increase in UV-B irradiation reaching the surface matters for life on Earth. One of the biggest concerns has been an increased risk of skin cancer (as well as skin damage and aging).2 This is because UV-B irradiation can damage skin DNA.
Since the 1980s, the world has achieved rapid progress: the near-elimination of ozone-depleting substances and the trend toward recovering the ozone layer are among the most successful international environmental achievements to date.
Several studies have estimated that millions of excess skin cancer cases have been avoided due to the Montreal Protocol and its follow-up treaties.3